Tianjin EURPING makes an exciting debut in BIOCHINA2024
BIOCHINA2024 was successfully held from March 14th to 16th at the Suzhou International Expo Center. The conference invited 94 conference chairpersons, expert committee members, and planners to jointly build the content of the Industry and Technology Forum; Invited 839 speakers to jointly create 128 forums, 3 closed door meetings, and 55 brainstorming case sharing sessions. During the same period, BIOPartnering has achieved nearly 5000 one-on-one meetings and negotiations.BIOCHINA 2024 invited 500+exhibiting institutions to jointly organize a 50000m2 bio industry exhibition, featuring five major professional exhibition halls: Excellent Brands, International Services, Raw Materials/Reagents/Consumables, Instruments and Equipment, and Comprehensive Hall, serving nearly 30000 professional visitors and 1000 professional purchasers, creating value for the co construction of the bio pharmaceutical industry supply chain.
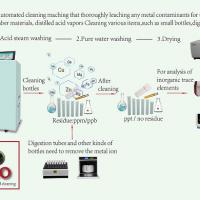
Tianjin EURPING brings GMP washer and pharmaceutical factory specialized laboratory bottle washing machine Q950PM to the exhibition, mainly to solve the cleaning pain points, cleaning plans, and cleaning validation risk assessment in current pharmaceutical production, helping pharmaceutical production units develop safer and more efficient standardized cleaning plans, making drug production safer and cleaner.
Exciting Review
Booth number: F3 Hall F008


The GMP washer can be used to clean key components in pharmaceutical workshops, such as fermentation tanks, rotary bottles, stoppers, silicone tubes, syringes, etc. All materials of the equipment are pharmaceutical grade materials, which comply with cGMP standards. The basket is measured according to the size of the customer's equipment and made in a customized manner.
Laboratory bottle washing machine Q950PM, used for cleaning and drying laboratory vessels such as volumetric flasks, test tubes, triangular flasks, test tubes, pipettes, culture dishes, and injection bottles. Siemens control system, cleaning data can be recorded, queried, exported, and printed, and the software meets GMP standard requirements.
 English
English Chinese
Chinese